Recombinant Antibodies
 |
|
Section 1. |
What is a recombinant antibody?
|
Section 2. |
GeneTex’s Recombinant Monoclonal Antibody Production Initiative
|
Section 3. |
Why GeneTex’s rabbit-based HL clones are a better choice.
|
Section 4. |
IVD-related Applications for GeneTex’s Recombinant Antibodies
|
Section 1. What is a recombinant antibody? |
|
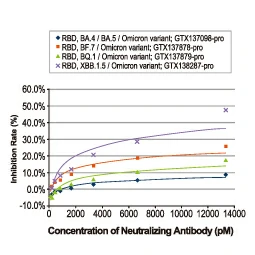
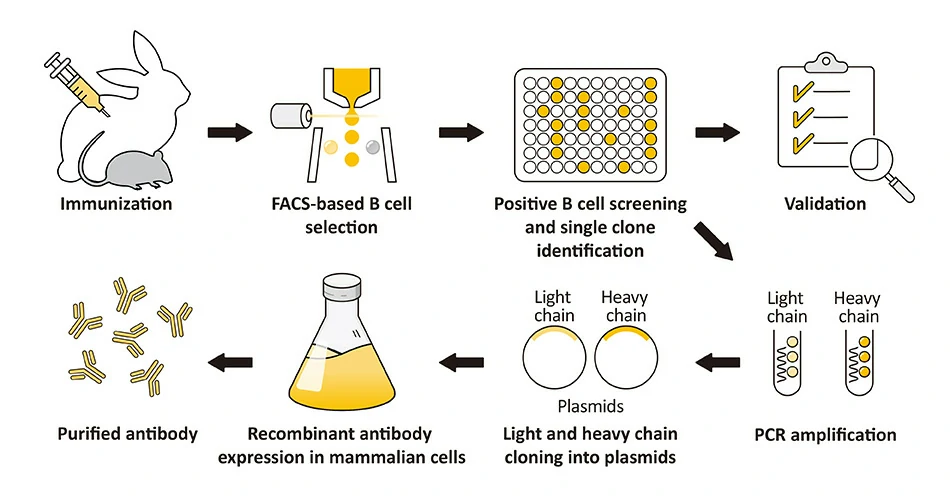
A recombinant antibody (rAb) is generated from the cloned, in vitro-expressed heavy and light chains of a selected monoclonal antibody obtained through classical hybridoma methodology, display strategies alone or in combination, sorted single B cells from immunized animals, or by one of many other techniques. The fact that it is cloned means the rAb is defined by its primary sequence, which can be engineered to optimize affinity and be expressed in different binder formats. The numerous advantages of rAbs have been well-documented, with consistency of performance being of paramount significance. GeneTex’s rAb protocol employs a multi-parameter FACS-based approach to isolate antigen-specific IgG+ memory B cells from an immunized animal, with subsequent cloning of the antibody variable-region genes into an IgG backbone and expression in mammalian cells (Fig. 1) (1). This protocol is very rapid and can be completed in weeks, and also affords the opportunity to identify antibodies with diverse capabilities in various applications. Importantly, it allows cloning of the heavy and light chains from the same B cell, thereby preserving natural pairing. And once cloned, the supply of a given rAb is inexhaustible with exceptional reproducibility. |
|
(A) |
 |
| (B) |
 Figure 1. (A) The GeneTex recombinant rabbit monoclonal antibody workflow (Starkie et al. (2016)). (B) Advantages of recombinant antibodies. |
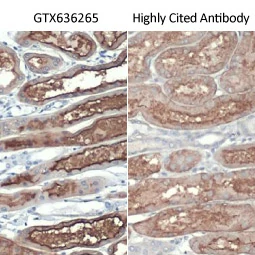
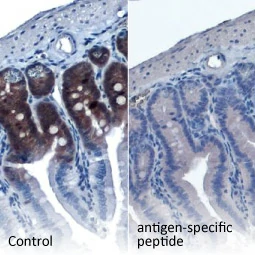
| One outstanding advantage of this protocol is that the suitability of the individual clones for desired applications can be tested during screening. For example, GeneTex’s Iba1 rabbit recombinant antibody [HL22] (GTX635363) detects Iba1, a protein commonly used as an immunohistochemical marker of both quiescent and activated microglia. In its development and production, both paraffin- and frozen-IHC analyses (IHC-P, IHC-Fr, respectively) were conducted in the first screening process to identify the best clones for these applications, as shown below (Fig. 2). The clones were compared to a highly cited, market-leading commercial antibody to gauge their performance. This strategy provides us with valuable perspective on a clone’s market competitiveness during development. In addition, our recombinant antibody team has successfully expressed the antigen-binding regions of this antibody in the context of both mouse and rat IgG backbones, thus extending flexibility for multiplexed staining. |
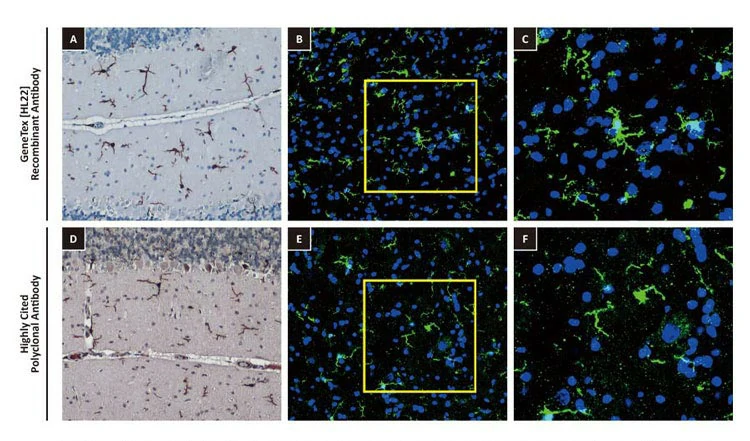
Figure 2. GeneTex's recombinant rabbit monoclonal Iba1 antibody [HL22] (GTX635363) is superior to a competitor's highly cited rabbit polyclonal antibody for both IHC-P (panels A vs. D) and IHC-Fr (panels B, C vs. E, F). |
|
Another new recombinant rabbit monoclonal reagent is GeneTex’s RAS (G12D mutant) antibody [HL10] (GTX635362) (Fig. 3). It demonstrates a robust signal and specificity for the G12D mutation in wild-type (WT) and KRAS G12D mutant pancreatic tumor tissue sections (Fig. 3A) and in established cell line lysates (Fig. 3B), respectively. This RAS (G12D mutant) antibody (GTX635362) is the first commercial recombinant version that shows exceptional specificity by IHC-P for this key tumorigenic mutant protein on sequence-verified human pancreatic tumor samples. |
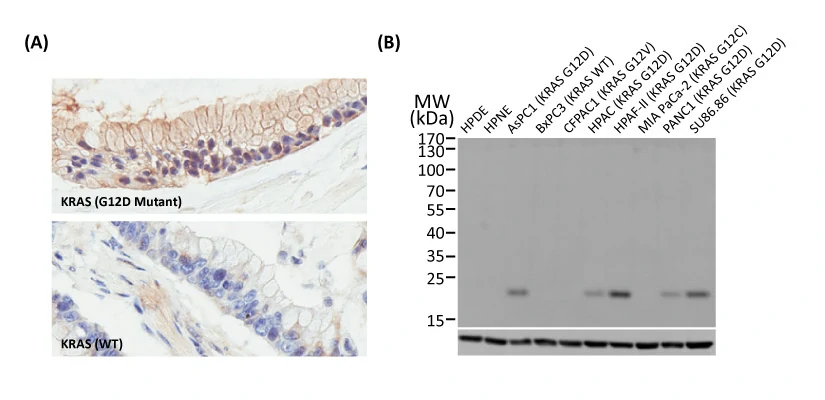
Figure 3. (A) GeneTex's recombinant rabbit RAS (G12D mutant) antibody [HL10] (GTX635362) is sensitive and specific for the RAS G12D mutation by IHC of a KRAS G12D mutant pancreatic tumor tissue section (top) compared to a wild-type KRAS section (bottom). (B) The antibody is sensitive and specific for the RAS G12D mutation by WB of extracts from wild-type and mutant KRAS-confirmed human pancreatic cell lines. GAPDH for the loading control was detected by GTX100118. Lane1: HPDE. Lane2: HPNE. Lane3: AsPC1 (KRAS G12D). Lane4: BxPC3 (KRAS WT). Lane5: CFPAC1 (KRAS G12V). Lane6: HPAC (KRAS G12D). Lane7: HPAF-II (KRAS G12D). Lane8: MIA PaCa-22 (KRAS G12C). Lane9: PANC1 (KRAS G12D). Lane10: SU86.86 (KRAS G12D). |
|
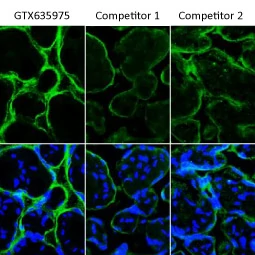
As mentioned above, the antigen-binding regions of a recombinant antibody can be inserted into various host IgG backbones or expressed in different binder formats (e.g., Fab fragments or scFvs). Here, GeneTex’s cloned TSG101 mouse monoclonal antibody (GTX70255) was converted to a rabbit IgG backbone (GTX635396) with preservation of performance. No cross-reaction was observed when the converted TSG101 rabbit IgG recombinant antibody was used in combination with an anti-mouse IgG secondary antibody (Fig. 4). |
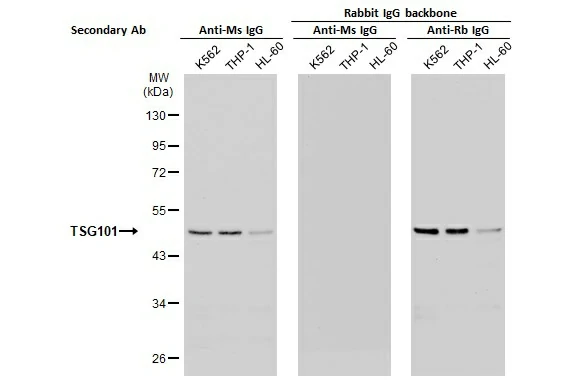
Figure 4. Backbone switch from a cloned mouse IgG antibody to a rabbit IgG antibody. |
Literature:
Flyer - Recombinant Abs
Brochure-Recombinant Antibodies
Reference:
|
Section 2. GeneTex’s Recombinant Monoclonal Antibody Production Initiative |
|
GeneTex’s production focus is the creation of novel recombinant rabbit monoclonal antibodies validated using enhanced protocols defined by the International Working Group for Antibody Validation (IWGAV). Over the last decade, studies published in many of the top scientific journals have clearly described the significant impact of poorly validated antibody reagents on the reproducibility of biomedical research. The unfortunate reality is that the commercial antibody market is compromised by a substantial subset of products that have been demonstrated to be nonspecific. This means that there are two major issues that must be addressed in order to improve the overall quality of the commercial antibody market and therefore the integrity and reproducibility of the biomedical research dependent on antibodies. The first is more widespread adoption of production platforms that emphasize recombinant antibody technology (please see “Section 1: What is a recombinant antibody?”) while the second involves incorporation of improved and more coherent validation strategies. GeneTex is making major strides in both of these endeavors. With the goal of providing better products for its customers through innovative technology, GeneTex has now shifted all of its new production to recombinant rabbit monoclonal antibodies (please see “Section 3: Why GeneTex’s rabbit-based HL clones are a better choice.”). Fully recombinant antibodies are defined by their primary sequence, their consistent performance and supply (issues that plague traditional polyclonals and hybridoma-based monoclonals with regard to lot-to-lot variability), and their ability to be engineered (i.e., backbone switching) to meet researcher requirements. These benefits may even evoke more favorable impressions by grant review and journal editorial committees. Other outstanding advantages of recombinant rabbit antibodies stem from the favorable biological characteristics of the rabbit immune system itself. In conjunction with its pivot to recombinant antibody production, GeneTex’s second commitment to antibody reliability is its incorporation of enhanced validation protocols, based on the IWGAV proposal mentioned above, into its manufacturing and quality assurance (QA) workflow (1). This involves evaluation of each antibody using at least one, and preferably more, of five validation “pillars” defined in the IWGAV study. GeneTex’s version of the IWGAV plan follows the five standard strategies and includes (1) Knockout/Knockdown; (2) Comparable Antibodies; (3) Immunoprecipitation followed by Mass Spectrometry (IP/MS); (4) Biological and Orthogonal Validation; and (5) Recombinant Protein Expression (Fig. 1). The company refers to this as its “5+1” Pillar Plan when referencing new recombinant antibody production, with the “+1” designation referring to the pre-validation that is inherent in the application-specific assessment that occurs with clone selection during the recombinant antibody production process (please see “New Validated Recombinant Monoclonal Antibodies from GeneTex” below). The new recombinant rabbit monoclonal antibodies that have been generated and evaluated using the production and validation approaches described above (these products are identified by “HL” in their clone designations) are just the beginning. GeneTex is also working to replace its successful polyclonals with new recombinants. The company envisions the “HL” clones representing the highest level of GeneTex validation and manufacturing capability. Antibody fidelity, application-dependent reliability, and performance and supply consistency are crucial for ensuring research integrity. GeneTex joins other reputable companies in leveraging recombinant antibody technology and enhanced validation to create antibodies that researchers can trust. |
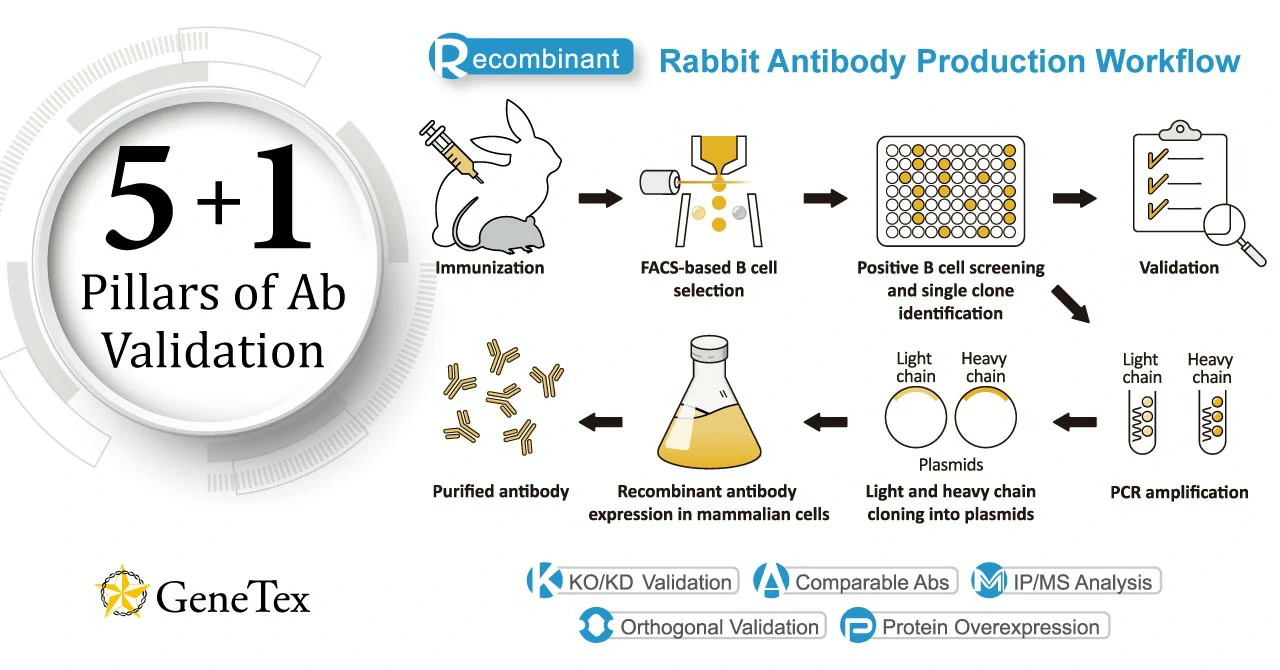
Figure 1. GeneTex’s “5+1” Pillar Plan for recombinant antibody validation. |
|
New Validated Recombinant Monoclonal Antibodies from GeneTex |
![]() Knockout / Knockdown Validation
Knockout / Knockdown Validation
Complete removal or significant reduction of the endogenous signal following genetic strategies involving genome editing or RNA interference, respectively.
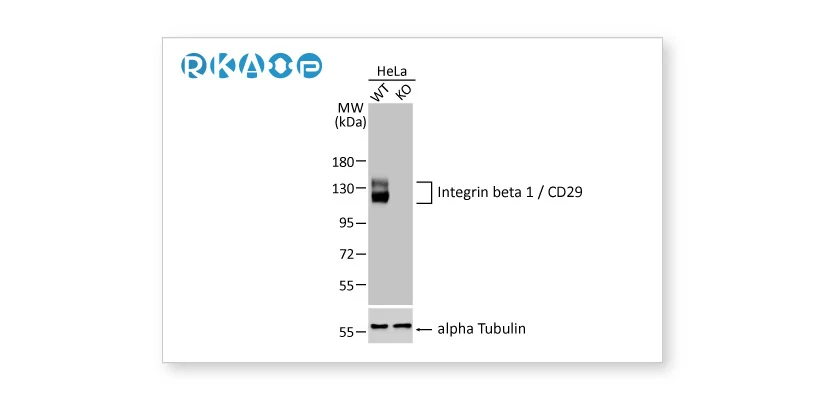 Integrin beta 1 / CD29 antibody [HL1255] (GTX636657) Integrin beta 1 / CD29 antibody [HL1255] (GTX636657) |
Independent antibodies against the same target but to different epitopes, often including distinct samples with different target protein expression levels.
|
|
![]() Biological and Orthogonal Validation
Biological and Orthogonal Validation
Alterations in the endogenous level of the target protein in accordance with specific preparation conditions corresponding to defined biological characteristics, or comparison between antibody-dependent and -independent methods.
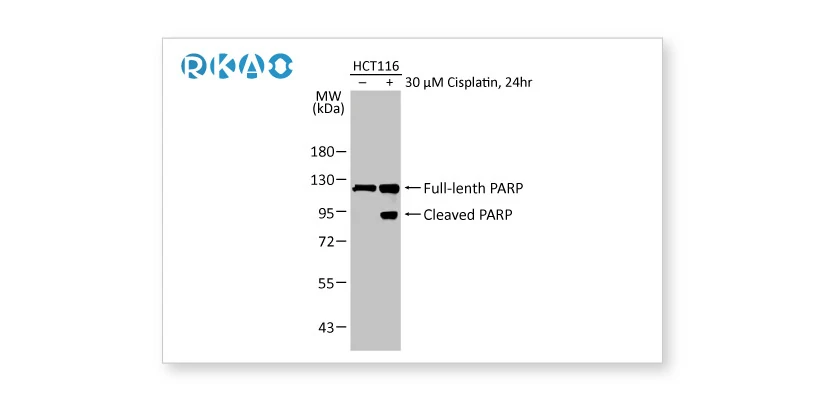 PARP antibody [HL1364] (GTX636804) PARP antibody [HL1364] (GTX636804) |
Tagged target proteins overexpressed in transfected cells are used as a positive control for validation.
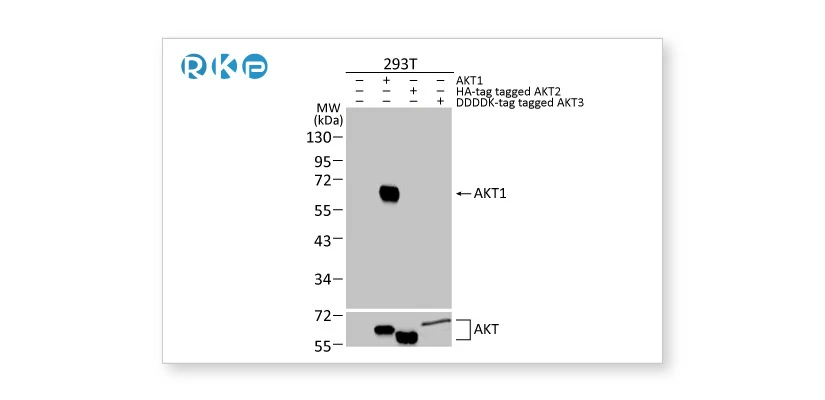 AKT1 antibody [HL1142] (GTX636413) AKT1 antibody [HL1142] (GTX636413) |
![]() Recombinant Antibody
Recombinant Antibody
Antibody is generated using recombinant technology.
 Gli1 antibody [HL247] (GTX635619) Gli1 antibody [HL247] (GTX635619) |
Reference:
|
Section 3. Why GeneTex’s rabbit-based HL clones are a better choice. |
|
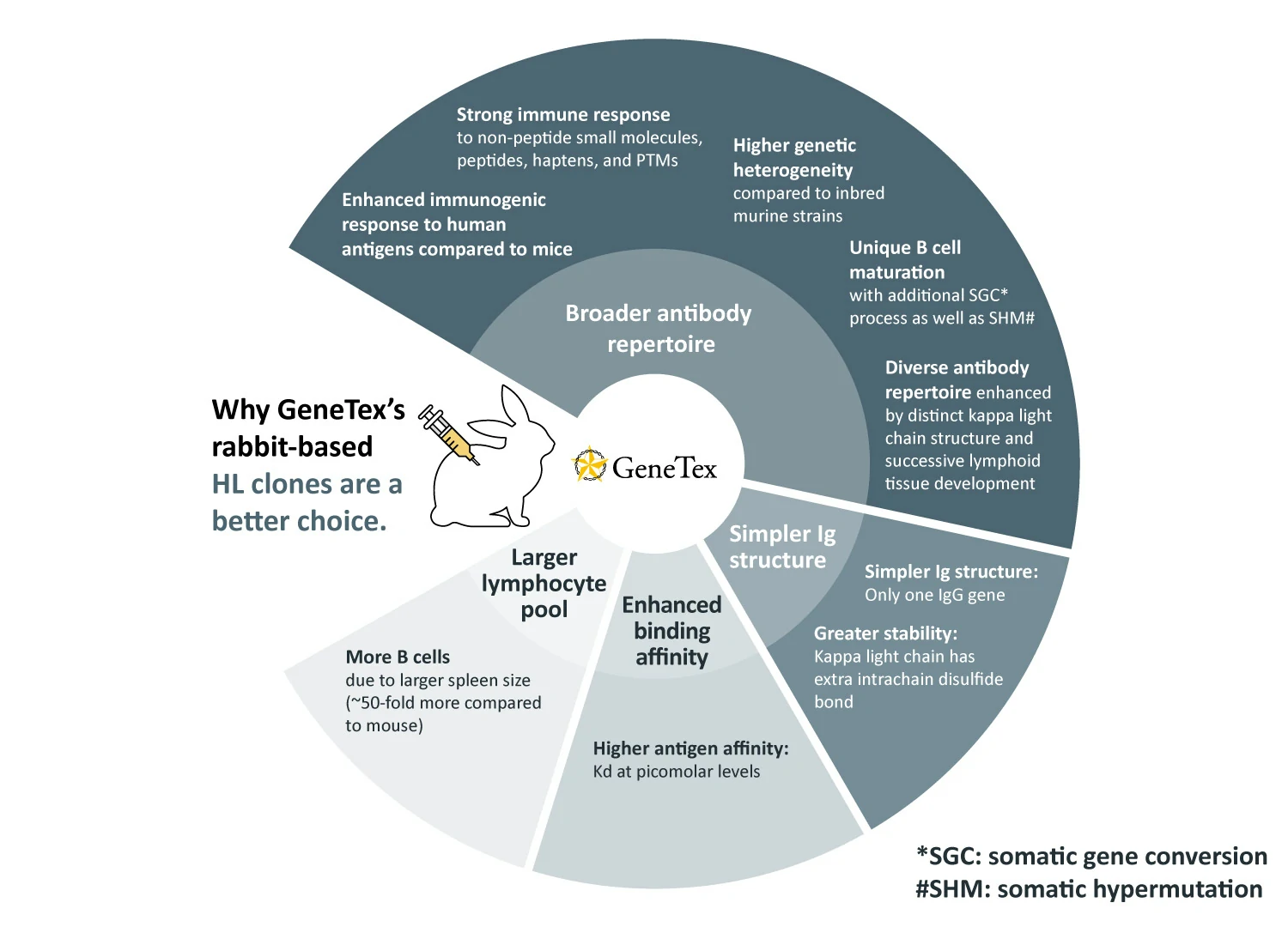
GeneTex’s recombinant rabbit monoclonal antibody production process is the platform for all new rabbit-based antibody generation (1). These products are distinguished by the “HL” designation in their respective clone numbers (e.g., “[HL1089]”). The rabbit, and specifically its immune system, is known to confer the following outstanding benefits for monoclonal antibody creation: broader antibody range, less complex immunoglobulin (Ig) structure, higher binding affinities, larger pool of lymphocytes to select antibodies (2). Each of these advantages is described in more detail below:
1. Broader antibody repertoire:
2. Simpler Ig structure:
3. Enhanced binding affinity:
4. Larger lymphocyte pool:
|
 |
|
GeneTex’s Well-validated HL Clone Antibodies |
|
|
|
||||||||||||
|
|
|
||||||||||||
References:
- PLoS One. 2016 Mar 29;11(3):e0152282. doi: 10.1371/journal.pone.0152282. eCollection 2016.
- Am J Transl Res. 2011 May 15;3(3):269-74. Epub 2011 Apr 23.
- Comp Immunol.2006;30(1-2):137-53. doi: 10.1016/j.dci.2005.06.017.
- Immunogenetics. 2016 Feb;68(2):83-107. doi: 10.1007/s00251-015-0868-8.
- Exp Mol Med.2017 Mar; 49(3): e305. doi: 10.1038/emm.2017.23.
|
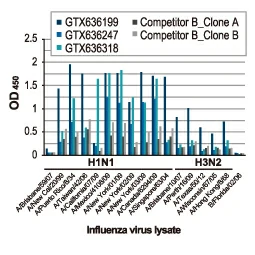
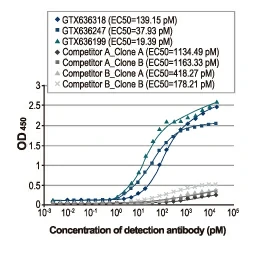
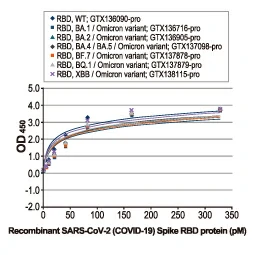
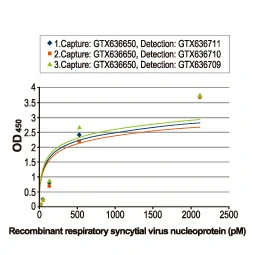
Section 4. IVD-related Applications for GeneTex’s Recombinant Antibodies |
Advantages:
- Superior Quality
- Performance Consistency
- Bulk Production (gram quantities)
- International QMS Certifications:
|
|
|
 |
 |
- Production in QMS IVD Laboratory
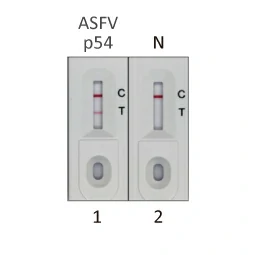
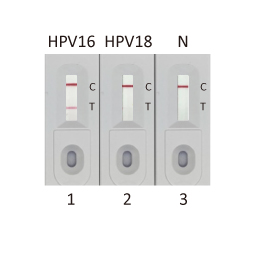
- Validated Antibody Pairs for IVD
- Product Development and Services
|
IVD-related Services: |
-Development-Services.webp) |
 |
|
|
|
||||||||||||
|
|
|
||||||||||||
-Development-Services.webp) |
 |
|
|
|
||||||||||||
|
|
|||||||||
 |
     |
 |
 |
 |
 |
 |
 |
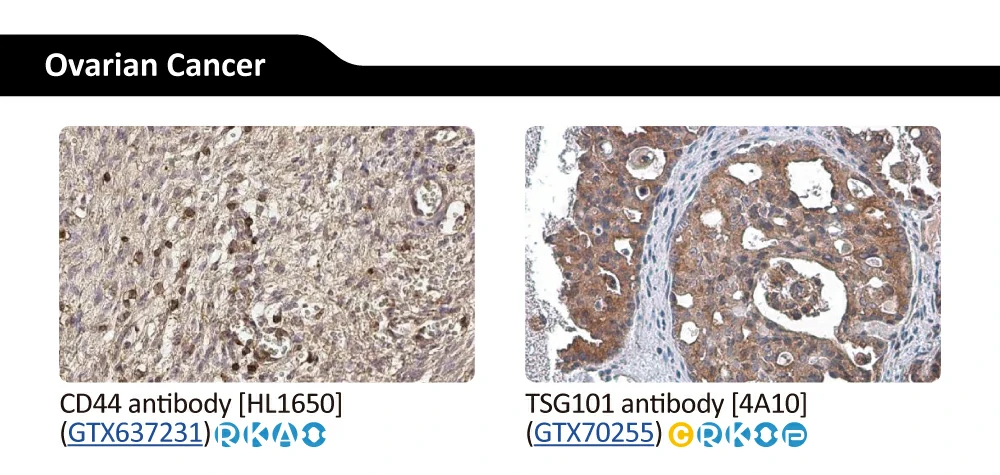 |
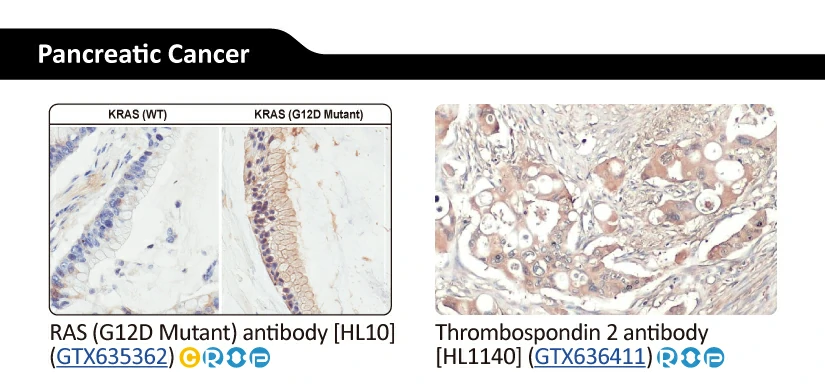 |
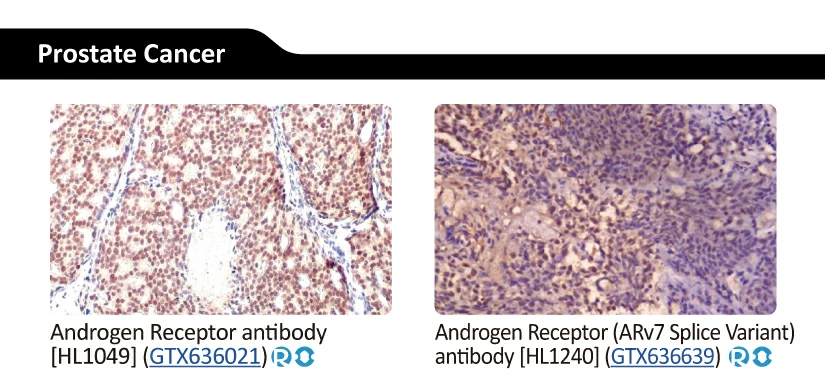 |
 |
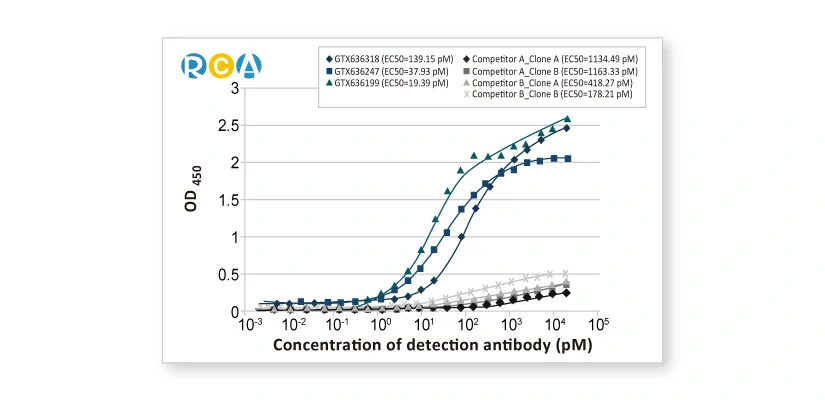
-Development-Services-2.webp)
.webp)
.webp)
.webp)
.webp)
-Development-Services-2.webp)