The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), previously known as 2019 Novel Coronavirus (2019-nCoV), is a positive-sense, single-stranded RNA virus that causes the potentially lethal COVID-19 respiratory tract infection. This new virus belongs to the genus Betacoronavirus, which also includes SARS-CoV and MERS-CoV.
GeneTex is proud to offer an extensive line of research antibodies and proteins to support the study of SARS-CoV-2, several of which were validated using virus-infected cell lysates. Please see the highlighted products below or click the button to see more product information.




|
|
![SARS-CoV / SARS-CoV-2 (COVID-19) spike antibody [1A9] (GTX632604)](/upload/media/research/Infectious-Diseases/COVID-19/GTX635654.jpg) |
|
Spike S1 antibody [HL6] (EC50: 0.552 nM)
|
|
GTX635654
|
|
 
|
|
|
|
 |
|
Nucleocapsid antibody [HL5511] (EC50: 0.014 nM)
|
|
GTX635689
|
|
  
|
|
|
| |
|
|
|
 |
|
Nucleocapsid ELISA Pair [HL5511 / HL448]
|
|
GTX500045
|
|
|
 |
|
Nucleocapsid ELISA Pair [HL5410 / HL455-MS]
|
|
GTX500042
|
|

|
|
|
|
| |
|
|
The first case of COVID-19 was detected in December, 2019 in Wuhan, China, and has now been declared a pandemic (1). With SARS-CoV-2 now reaching pandemic status, researchers and clinicians have been working furiously to learn more about the virus’s biology and pathogenesis as well as how to treat the more clinically aggressive COVID-19 cases.
In their study published very recently in Cell, Hoffmann et al. confirm findings reported by Zhou et al. that angiotensin-converting enzyme 2 (ACE2) is the cellular receptor for SARS-CoV-2, as it is for SARS-CoV (2, 3). In addition, they identify the serine protease TMPRSS2 as a critical factor in the priming of the SARS-CoV-2 spike (S) protein, an essential step for viral entry into host cells through fusion of the viral and cellular membranes. The authors also demonstrate that the serine protease inhibitor camostat mesylate, an agent that has already seen clinical application as a treatment for chronic pancreatitis in Japan, is able to interfere with SARS-CoV-2 infection of lung cells.
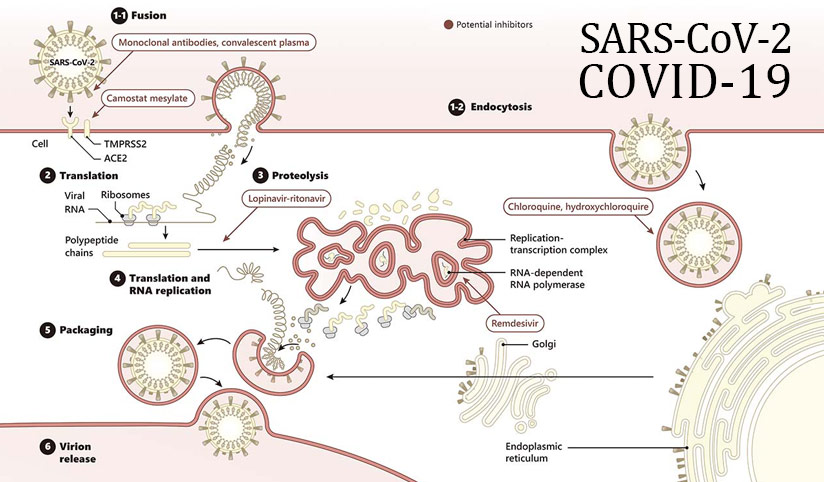
Adapted from Science 367(6485), 1412-1413 (2020).
|
To further expedite the identification of agents with activity against SARS-CoV-2, the WHO’s SOLIDARITY study will assess the potential utility of four different classes of clinically known drugs or drug combinations against the virus (4). The four arms include the viral polymerase inhibitor remdesivir, the anti-malarial agents chloroquine and hydroxychloroquine, the HIV protease inhibitors lopinavir and ritonavir, and lopinavir/ritonavir with interferon-beta. This study, which is one of many ongoing trials that are reviewing more than a dozen possible therapies, is stripped down to accelerate data acquisition and be accessible to physicians everywhere. The central focus of many of these trials is to evaluate existing agents with acceptable safety profiles (e.g., the serine protease inhibitor camostat mesylate) that are amenable to repurposing for use against SARS-CoV-2. In addition, compounds previously found to have activity against SARS-CoV, MERS-CoV, or other viruses are being reexamined, as is the use of convalescent plasma from recovered COVID-19 patients and monoclonal antibodies targeting viral components (5). The singular determination to fight this virus as a world community is now widely appreciated as the only path to eventual success.

![]()
![]()
![]()
![]()


![SARS-CoV-2 (COVID-19) Spike S1 antibody [HL6]](/upload/media/research/Infectious-Diseases/COVID-19/GTX632604.jpg)
![SARS-CoV / SARS-CoV-2 (COVID-19) spike antibody [1A9] (GTX632604)](/upload/media/research/Infectious-Diseases/COVID-19/GTX635654.jpg)
![SARS-CoV-2 (COVID-19) nucleocapsid antibody [HL249]](/upload/media/research/Infectious-Diseases/COVID-19/GTX635692.jpg)

![ACE2 antibody [N1N2], N-term (GTX101395)](/upload/media/research/Infectious-Diseases/COVID-19/GTX135589.jpg)
![TMPRSS2 antibody [N2C3] (GTX100743)](/upload/media/research/Infectious-Diseases/COVID-19/GTX135467.jpg)





